Learning Outcomes
i. Master the concept of ion movement in electrolytic cells, understanding the direction of migration of cations and anions.
ii. Identify the role of the external electrical energy source in driving ion movement.
iii. Recognize the significance of ion movement in maintaining electrical neutrality and facilitating redox reactions.
iv. Apply the principles of ion movement to explain the functions of various electrochemical devices, such as electrolysis and batteries.
Introduction
In the fascinating realm of electrochemistry, where chemical energy transforms into electrical energy, the movement of ions plays a pivotal role in orchestrating the symphony of reactions. This lesson will delve into the intricacies of ion migration in electrolytic cells, empowering you to decipher the direction of cations and anions and appreciate their significance in driving chemical transformations.
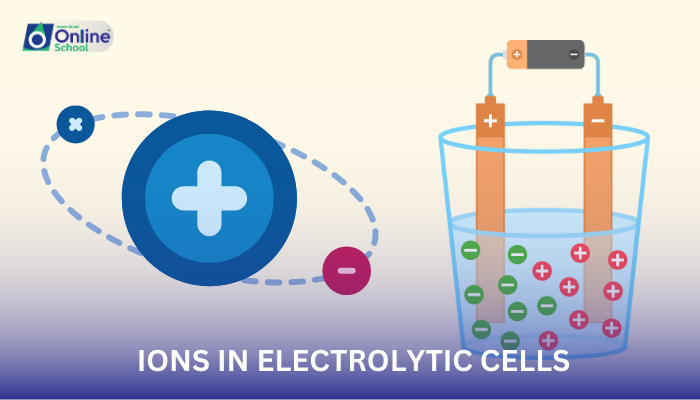
i. Direction of Ion Movement: A Tale of Electrostatic Forces
The movement of ions in an electrolytic cell is governed by the interplay of electrostatic forces and the external electrical energy source. Cations, the positively charged ions, are attracted to the cathode, while anions, the negatively charged ions, are attracted to the anode. This movement of ions is facilitated by the conductive electrolyte solution.
ii. The Role of External Electrical Energy: A Guiding Force
The external electrical energy source plays a crucial role in driving the movement of ions. By applying a voltage, the energy source creates an electric field that exerts a force on the charged ions, directing them towards their respective electrodes.
iii. Maintaining Electrical Neutrality: A Balancing Act
The movement of ions in an electrolytic cell is carefully balanced to maintain electrical neutrality. The salt bridge, a porous barrier separating the anode and cathode compartments, allows the passage of ions while preventing bulk mixing of the electrolyte solutions. This ensures that the overall charge in each compartment remains neutral.
iv. Significance of Ion Movement: A Driving Force for Reactions
The movement of ions is essential for the occurrence of redox reactions in electrolytic cells. At the cathode, cations gain electrons, undergoing reduction, while at the anode, anions lose electrons, undergoing oxidation. These redox reactions are the heart of electrolysis, driving the non-spontaneous decomposition of compounds.
v. Real-World Applications: A Symphony of Electrochemical Processes
The principles of ion movement in electrolytic cells find applications in various electrochemical devices, including:
Electrolysis: The decomposition of compounds using electrical energy.
Batteries: Electrochemical cells that generate electrical energy through spontaneous redox reactions.
Electroplating: The deposition of a metal onto a cathode from an ionic solution.
The movement of ions in electrolytic cells is a fundamental aspect of electrochemistry, providing the driving force for redox reactions and enabling the conversion of chemical energy into electrical energy. Understanding the direction of ion movement, the role of external electrical energy, and the significance of ion movement in maintaining electrical neutrality empowers us to appreciate the intricate dance of ions that underpins various electrochemical processes.